Client Profile
The University of Birmingham is a leading global institution based in the UK, renowned for its research excellence and diverse academic programmes. With over 8,000 staff members, it offers a vibrant campus life that fosters innovation, inclusivity, and academic achievement.
Objectives
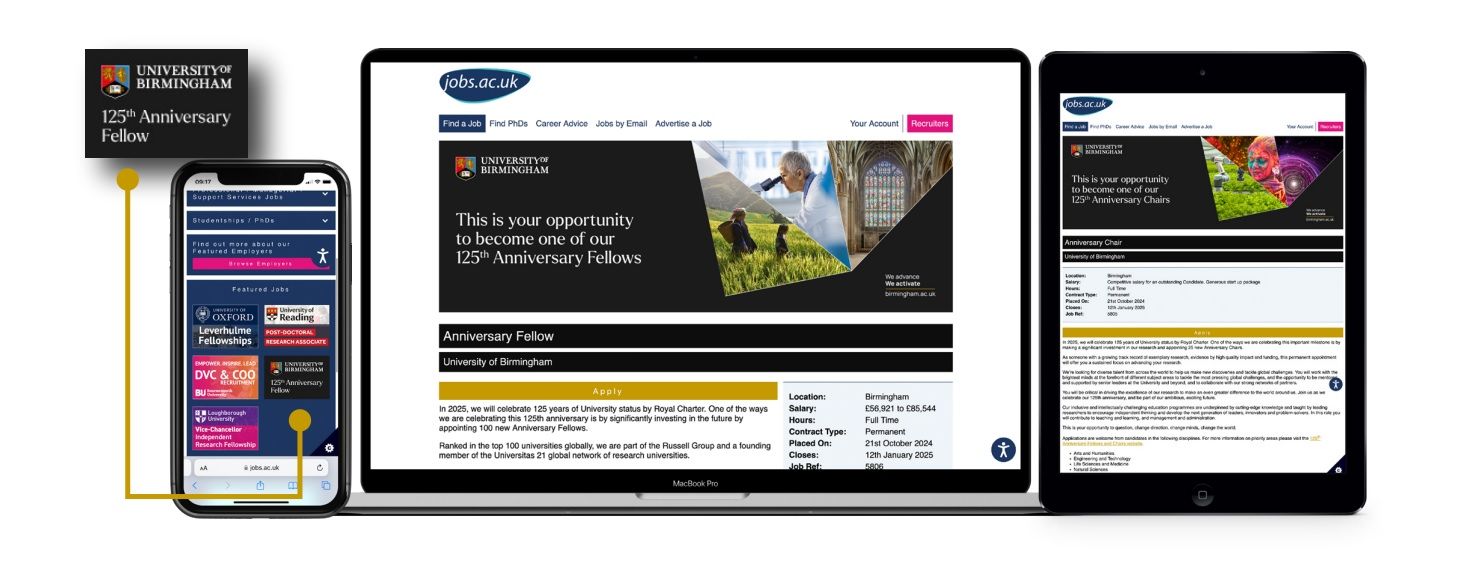
The University of Birmingham sought talented candidates to fill various positions. Focusing on their 125th Anniversary Fellows and Chairs campaign, read on to discover how we collaborated with them and the steps we took to support their vision and goals for the recruitment initiative.
Challenges faced/ reason for using jobs.ac.uk
We chose to advertise the 125th Anniversary Chairs and Fellows roles on jobs.ac.uk for several reasons. As a leading jobs board for higher education, the site effectively reaches highly qualified candidates actively seeking academic positions. Given the prestigious nature of these roles and the importance of our 125th anniversary, it is essential to attract exceptional talent from a range of research disciplines.
Additionally, several of our recently appointed anniversary researchers discovered the opportunities on jobs.ac.uk last year, which reinforced our decision to continue using this channel. This time, we have enhanced our approach with improved advertising placements and branding. The robust promotion tools enable us to create a strong and cohesive brand presence by placing our ads in various categories, maximising visibility among candidates who align with our priority recruitment areas.
Solutions Implemented
Both adverts have been listed as premium, multiple-post, composite adverts, as both calls are large recruitment drives aimed at hiring numerous candidates.
The links to each advert are provided below:
- https://www.jobs.ac.uk/job/DKG958/anniversary-fellow
- https://www.jobs.ac.uk/job/DKG952/anniversary-chair
Results and impact
To date, advertising through jobs.ac.uk has generated positive results for our recruitment efforts. We have received a strong response from a diverse pool of candidates, many with impressive backgrounds in impactful research. While jobs.ac.uk is a UK-based website, it effectively attracts a significant number of international candidates, which has been crucial for reaching global talent.
Overall, this campaign has enhanced the University’s profile as an attractive global destination for academic talent. I look forward to monitoring the campaign’s ongoing performance.
Client satisfaction feedback
Working with jobs.ac.uk has been a positive experience. What stood out was their deep understanding of the higher education landscape and their ability to tailor our advertisements to effectively reach the right audience. The support team have been responsive and proactive, offering valuable insights on optimising our postings.
Additionally, the analytics provided gave us a clear understanding of our reach and engagement. Overall, this collaboration has significantly contributed to our recruitment successes and has aligned perfectly with our aim to attract talent for our 125th anniversary initiative.


Share your comments and feedback